Side Effects
Side Effects
TECVAYLI® may cause side effects that are serious, life-threatening, or lead to death, including and neurologic problems.
Call your healthcare provider right away if you develop any of the signs or symptoms of CRS or listed below at any time during your treatment with TECVAYLI®:
Cytokine Release Syndrome (CRS). Signs and symptoms of CRS may include:

Fever
(100.4°F or higher)

Difficulty breathing

Chills

Dizziness or lightheadedness

Fast heartbeat

Feeling anxious

Confusion or restlessness

Headache

Increased liver enzymes in your blood
Step-up dosing may help reduce both the chance of getting CRS and the severity of it
TECVAYLI® activates your immune cells to help fight your disease, which can lead to CRS. Your provider will give you premedication and use step-up dosing to decrease the likelihood and severity of CRS. Pretreatment may also be needed for doses given after a dose delay.
Call your healthcare provider right away if you develop any of the symptoms listed below at any time during your treatment with TECVAYLI®.
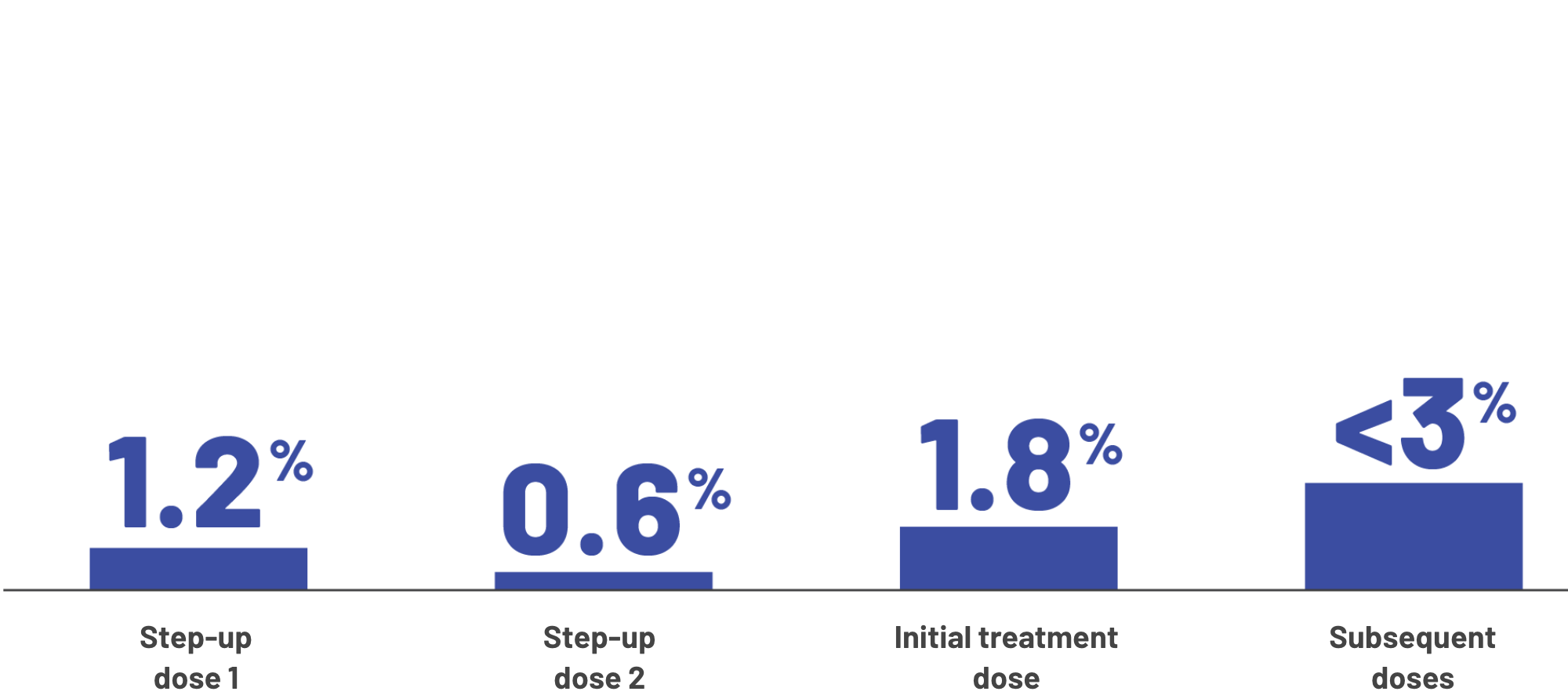
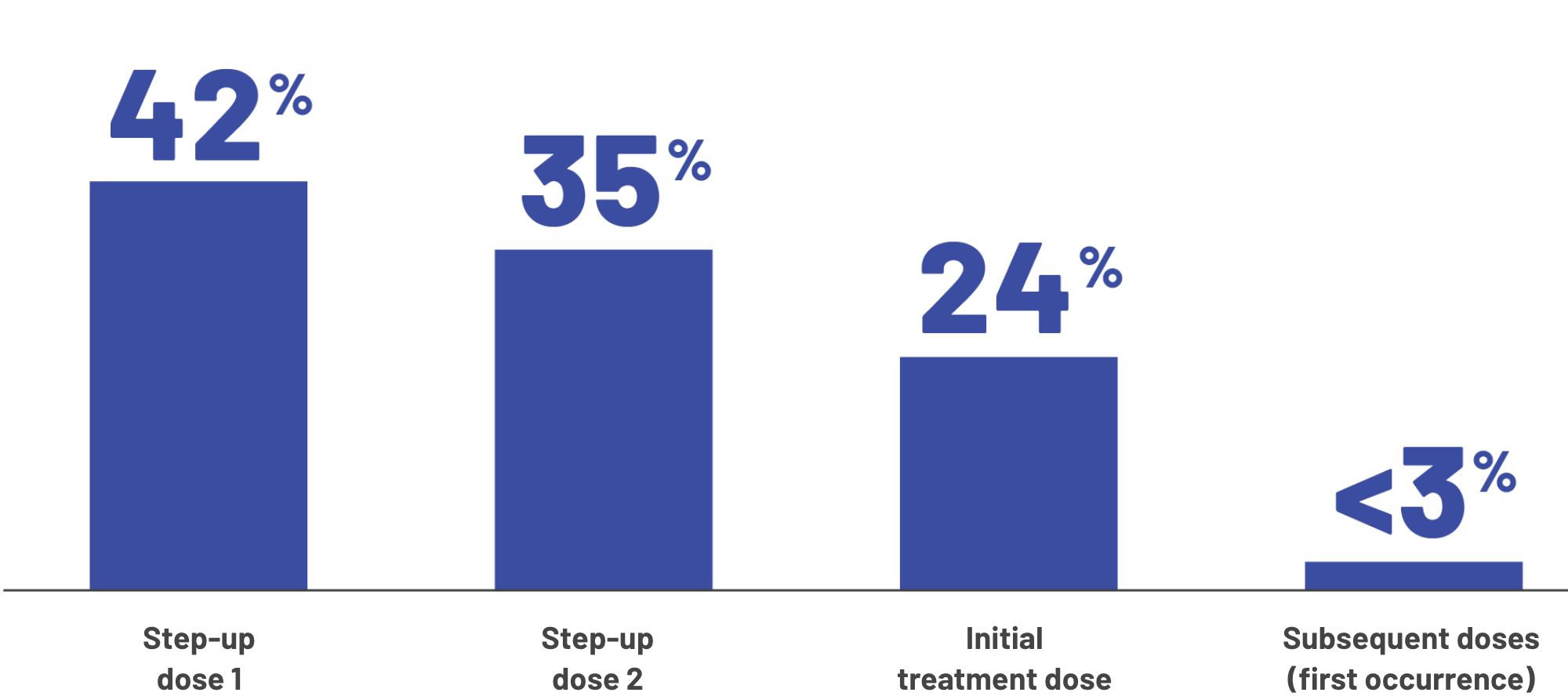
Most instances of CRS happened during the first 3 doses
Severity of CRS experienced by patients
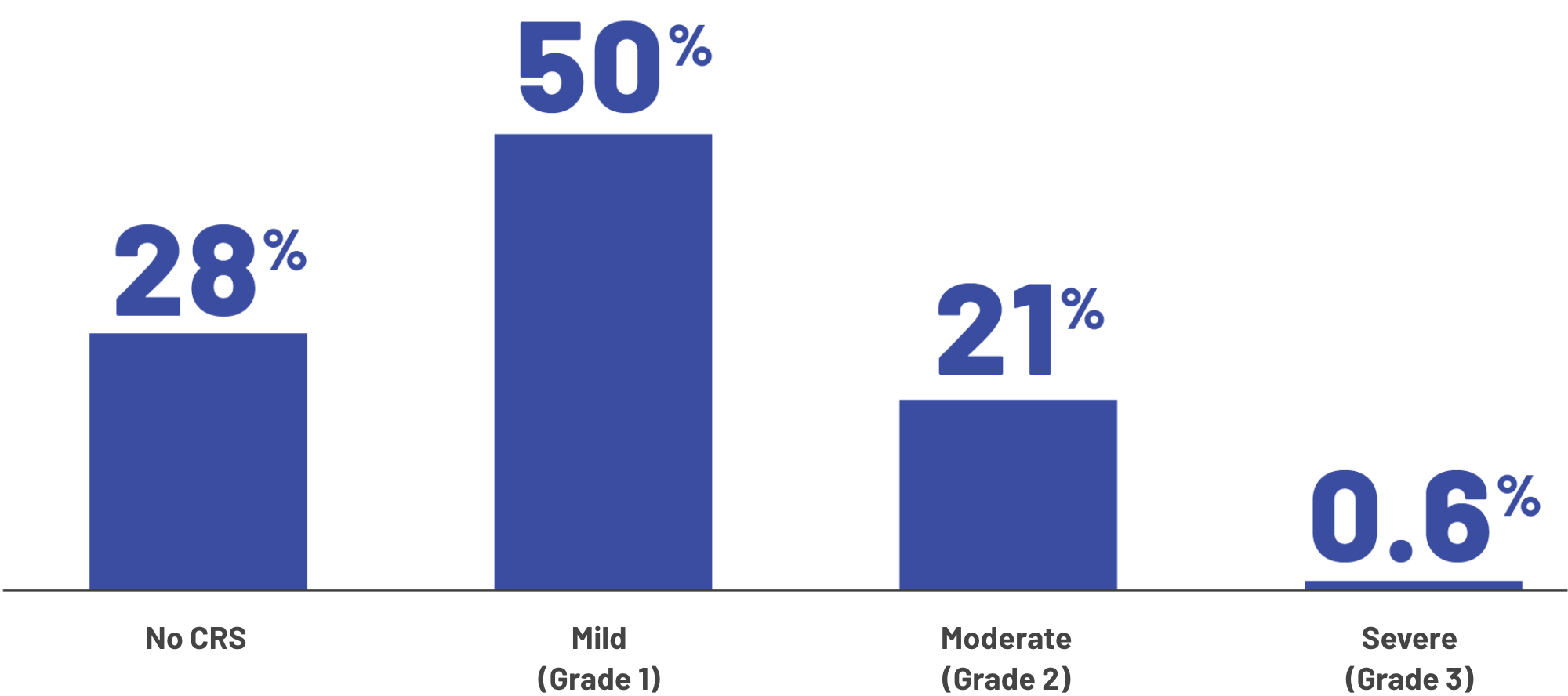
The median time to CRS occurring was 2 days (with a range of 1 to 6 days) after the most recent dose. The median time that CRS lasted was 2 days (with a range of 1 to 9 days).
Neurologic problems. Symptoms of neurologic problems with TECVAYLI® include:

Headache

Jerking movements

Rigid muscles

Feeling restless

Numbness and tingling (feeling like "pins and needles")

Confusion

Trouble speaking

Muscle spasms

Tremor

Double vision

Changes in
your handwriting

Problems walking

Muscle weakness in your body or face
Hearing loss
Burning, throbbing,
or stabbing pain
Understanding Neurologic Problems
Neurologic problems can occur with TECVAYLI®, including a specific type called immune effector cell-associated neurotoxicity syndrome (ICANS).
Severity of all neurologic problems experienced by patients
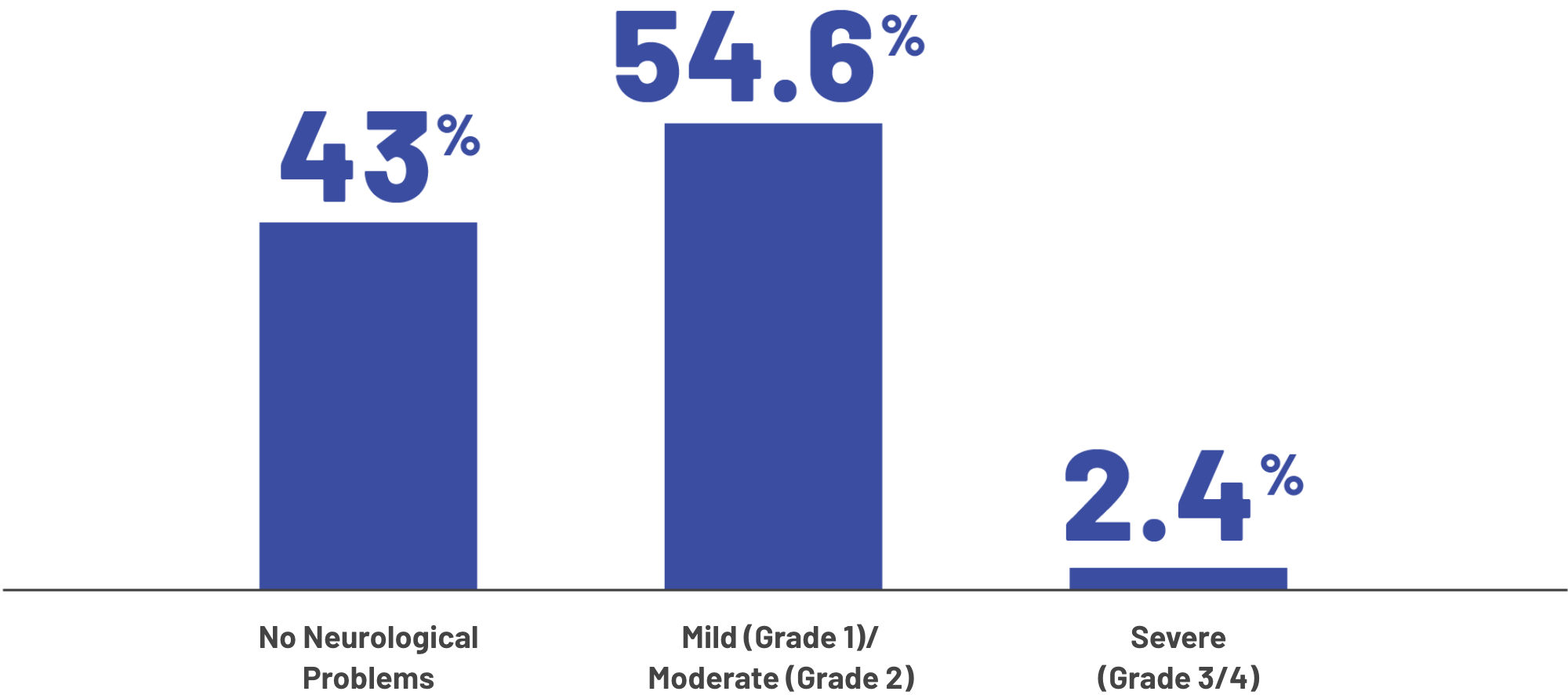
The median time to neurologic problems occuring was 4 days (with a range of 2 to 8 days) after the most recent dose. The median time that neurologic problems lasted was 3 days (with a range of 1 to 20 days).
Due to the risk of CRS and neurologic symptoms, you should be hospitalized for 48 hours after all doses of TECVAYLI® that are part of the “step-up dosing schedule.” The “step-up dosing schedule” is when you receive the first 2 doses of TECVAYLI®, which are called “step-up doses,” and then you receive the first “treatment dose” of TECVAYLI®. After “step-up dose 1” of TECVAYLI®, the dose of TECVAYLI® is increased. After “step-up dose 2,” the dose is increased again when you receive the first “treatment dose” of TECVAYLI®.
Your healthcare provider will monitor you for signs and symptoms of CRS and neurologic problems during treatment with TECVAYLI®, as well as other side effects and treat you as needed.
Do not drive or operate heavy or dangerous machinery during and for 48 hours after your TECVAYLI® “step-up dosing schedule” is completed, or at any time during treatment with TECVAYLI® if you develop new neurologic symptoms until the symptoms go away.
What are the possible side effects of TECVAYLI®?
TECVAYLI® may cause serious side effects, including:
See “What is the most important information I should know about TECVAYLI®?”
Liver problems. TECVAYLI® can cause liver problems that may lead to death. Increased bilirubin and liver enzymes in your blood are common with TECVAYLI® and can also sometimes be severe. These increases in liver enzymes can happen with or without you also having CRS. Your healthcare provider will monitor you for these problems before you start and during treatment with TECVAYLI®. Tell your healthcare provider if you develop any symptoms of a liver problem including:
tiredness
loss of appetite
pain in your right upper stomach area (abdomen)
dark urine
yellowing of your skin or white part of your eyes
Infections. Upper respiratory tract infections and pneumonia are common with TECVAYLI®. TECVAYLI® can cause bacterial and viral infections that are severe, life-threatening, or that may lead to death.
Your healthcare provider will monitor you for signs and symptoms of infection before and during treatment with TECVAYLI®.
Your healthcare provider may prescribe medicines for you to help prevent infections, and treat you as needed if you develop an infection during treatment with TECVAYLI®.
Tell your healthcare provider right away if you get a fever, chills, or any signs or symptoms of an infection.
Decreased white blood cell counts. Decreased white blood cell counts are common with TECVAYLI® and can also be severe. Fever sometimes also happens with low white blood cell counts and may be a sign that you have an infection. Your healthcare provider will check your blood cell counts before you start and during treatment with TECVAYLI®, and treat you as needed.
Allergic reactions and injection site reactions. TECVAYLI® can cause allergic reactions that can affect your whole body (systemic), and also cause injection site reactions.
Some people taking TECVAYLI® can develop symptoms of an allergic reaction that can affect their whole body and may include fever or a swollen tongue. Get medical help right away if you develop symptoms of an allergic reaction during treatment with TECVAYLI®.
Injection site reactions are common with TECVAYLI® and can include: redness, heat, swelling, bruising, bacterial skin infection (cellulitis), discomfort, blood collection under the skin at the injection site (hematoma), and rash. Tell your healthcare provider if you develop any severe injection site reactions.
Your healthcare provider may temporarily or permanently stop TECVAYLI® if you have any of the side effects listed above and they are severe.
The most common side effects of TECVAYLI® include:

Fever
Pain in your joints and muscles, back and chest muscles, and in your arms and legs
Tiredness and
weakness
Upper respiratory tract infections and pneumonia. See "Infections" above
Nausea

Headache
Diarrhea
The most common severe abnormal lab test results with TECVAYLI® include:

Decreased white blood cells, red blood cells, and platelets
These are not all the possible side effects of TECVAYLI®.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
Your healthcare team may change your treatment plan if you experience side effects.
TECVAYLI® is available only through the TECVAYLI® and TALVEY® Risk Evaluation and Mitigation Strategy (REMS) due to the risk of CRS and neurologic problems.
You will receive a Patient Wallet Card from your healthcare provider. Carry the Patient Wallet Card with you at all times and show it to all of your healthcare providers. The Patient Wallet Card lists signs and symptoms of CRS and neurologic problems.